IDERHA
is an open platform designed to enable connectivity, access, sharing, use and reuse of digital health data. Our policy recommendations will enhance regulatory and HTA decision making, facilitating access to digital health innovations for patients and health care professionals.

Transforming data into better healthcare
In recent years, there has been an exponential
growth in the generation of data that could be
harnessed for use in healthcare delivery and
research. These data include readouts generated
by digital technologies, patient reported outcome
and experience measures, and results from
clinical trials and routine clinical care. However,
accessing, integrating and analysing these data
to maximize their value for patient care and
research is extremely challenging.
Lung cancer as EU’s key priority
IDERHA will focus on lung cancer, which is
responsible for 20% of cancer-related deaths
in Europe and one of the key priorities of the
Europe Beating Cancer program.
The project will link and analyse diverse data
of a lung cancer patient’s journey: from early
screening of citizens at risk, to development of
lung cancer, to remote monitoring of late-stage
patients to enable better care in an at home
setting. Ultimately, the platform and policy
recommendations will be disease independent.
IDERHA (Integration of heterogenous Data and Evidence towards
Regulatory & HTA Acceptance)
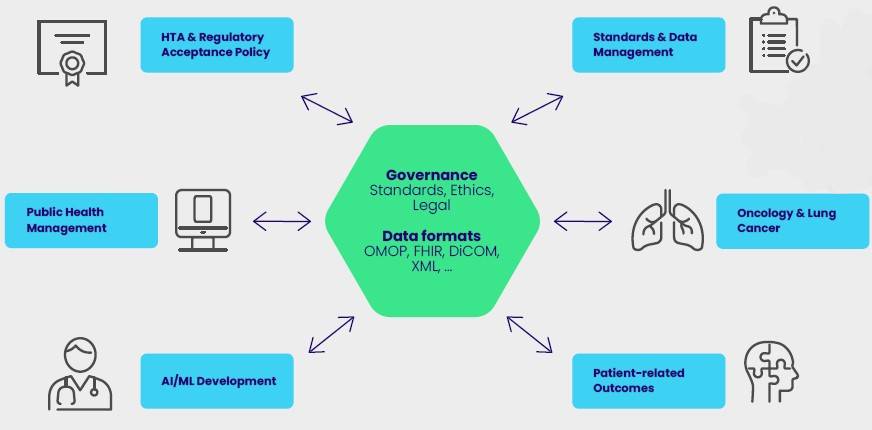
The project aims to create a scalable platform
for the seamless integration or linkage of
diverse data to support healthcare professionals,
patients, and researchers with new capabilities
to improve patient outcomes. The new platform
will be based on the development of common
standards and practices – reducing current
disconnected information silos. This enables more
personalized health care along the patient’s
journey via data-driven tools and solutions.
The consortium will, therefore, develop the first
pan-European health data space, and through
policy recommendations drive health data
access and the acceptability of heterogeneous
health research results for regulatory and Health
Technology Assessment decision-making.
Accelerate policy development
To enable patients and health care providers
to benefit from novel health solutions, IDERHA
also seeks to accelerate policy development.
Informed by patient needs, we will develop
consensus recommendations for fully compliant
health data access and sharing, as well as
for the acceptability of heterogeneous health
research results for regulatory and HTA decisionmaking.
This may lead to a framework for data
governance that can be used as a model globally.
Funding:
IDERHA is among the first projects funded
through the EU Innovative Health Initiative
(IHI), a public-private partnership (PPP)
between the EU and the European life science
industries. IDERHA’s total budget is € 42.7
mln (plus contributions from Associated
Partners from Switzerland and the UK).
Duration:
IDERHA will run for 5 years,
from 1 April 2023 – 31 March 2028.
More information:
info@iderha.org
‘IDERHA is a pioneering
project focussed on
creating health care
solutions that integrate
diverse health data, to
bring improved care and
treatments for patients.’
KEY FACTS
IDERHA leadership & management:
IDERHA is led by the Fraunhofer Institute for
Translational Medicine and Pharmacology
ITMP and Johnson & Johnson Medical GmbH,
part of Johnson & Johnson MedTech.
The management team consists of Dr. Philip
Gribbon (Fraunhofer ITMP) and Dr. Christian
Muehlendyck MD (Johnson & Johnson
MEDICAL GmbH).
Consortium partners:
33 academic, clinical, medtech,
pharmaceutical, IT and patient advocacy
organizations as well as public authorities
from across the EU and the world.
