Dénia Health Department belongs to the Marina Alta area, a region of the Valencian Community in Spain. This health department is managed by the Marina Salud concessionaire of the Ribera business group. Since 1997, Ribera has been a provider of public and private health services. With more than 7,000 professionals, its vocation is to transform the way of working and innovate in healthcare management. It has developed a healthcare model that is being studied as a success story at renowned international universities such as Harvard Business School or Berkeley.
Dénia Marina Salud Hospital (HDM) opened its doors in 2009 and has a population size of approximately 200,000 inhabitants during part of the year, while during the summer season its population tends to increase significantly due to the increase in tourists and residents, for all this, the population increases by more than 300,000 inhabitants during the high seasons.
HDM has a network of 34 primary care points spread throughout the Marina Alta region and a 206-bed hospital.
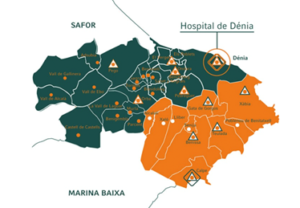
The HDM is the reference health center in Marina Alta, it has a broad portfolio of services and indicators in surgery and outpatient consultations, with delays well below the average for the Valencian Community.
We, in Dénia Health Department, serve an average of 5,000 patients a year, of which around 750 are new cases. The most frequent tumors among the population of the Marina Alta are breast cancer or those related to the digestive system, prostate, and lung, in that order.
In 2014, HDM became the first center in the world, outside of the United States, to earn HIMSS Davies Award level 7. This is an international award from the independent rating firm, HIMSS Analytics, that classifies hospitals according to the degree of integration of the EMR (Electronic Medical Record) of the Patient. Precisely this digitalization of the EMR is one of the pillars of joint work and collaboration with the iHelp project, for the development of Holistic Health Records (HHR) that allow nurturing of an AI system based on biomedical data.
The HDM team is carrying out a pilot study on lifestyle habits with the ability to increase risk factors for pancreatic cancer. The purpose of the HDM pilot is to study how risk factors and changes in lifestyle in a healthy population have a direct implication on the risk of developing pancreatic cancer compared to biomedical data collected from a group of cancer patients of the pancreas. Therefore, this pilot focuses on developing tools for predicting the risk of pancreatic cancer to prevent or reduce potential risk factors in the healthy population and help in clinical decision-making.
We have designed a study focused on the follow-up and monitoring of a group of healthy study subjects but who present a smoking habit as the main risk factor. To do this, starting in September 2022, we are going to carry out a 9-month monitoring and follow-up period, in which we will encourage and promote change and improvement in life habits with the cessation of the group’s smoking habit healthy to check how these changes affect the epigenetic profile of each study subject and other biomedical data collected in the pilot follow-up period.
These changes in the lifestyles of each individual will be enhanced through the subjects’ participation in the courses and workshops designed by the HDM team. The changes will be reinforced with a clinical follow-up plan and also through active notifications through electronic devices to be administered to the study subjects.
The collection of HHR data during the course of follow-up will be through the EMR of our hospital, analytical data, epigenetic profile, and the data collected by the electronic devices managed with the ultimate goal that our AI platform is able to help prevent potential risk factors of the healthy population and support in clinical decision-making.
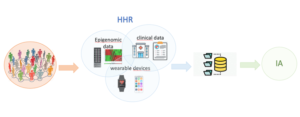
For the development of analytical models and decision support tools, data records, questionnaires, and electronic devices will be used to monitor each participant.